ISPAD 2022: what we know about type 1 diabetes may be upside down
In October 2022, I was fortunate enough to attend the Annual Conference of the International Society for Pediatric and Adolescent Diabetes (ISPAD) as a #dedoc° voice. Whilst there, I learned that there’s a controversy within the science of type 1 diabetes which challenges its cause.
The conventional thinking on the cause of type 1 diabetes begins with what we can observe, namely the immune system. Generally speaking, in people with type 1 diabetes, we see auto-antibody markers. In other words, the body’s immune system is attacking the pancreas and, specifically, the beta cells.
Environmental factors and type 1 diabetes: how big a role do they really play?
The idea often put forward is that an “environmental factor” interacts with the body somehow, and the immune system responds to what it believes is a threat. As the immune system is a learning system, it remembers the threat and is prepared to attack it in the future. The theory is that the threat looks similar to the beta cells in the body and so the immune system begins attacking them, leading to depletion and type 1 diabetes.
That environmental factor might be a virus or it could be gluten causing a “leaky gut”, thereby exposing the immune system to food particles and confusing it. Professor Bart Roep is suggesting that pinning the cause of type 1 diabetes on the “environmental factor” is missing the mark.
Professor Roep gave his talk during the first session of the first day and set quite the benchmark. He is looking at how we approach cancer treatment and whether the lessons learned there can be used for the treatment of type 1 diabetes. Rather than seeing type 1 diabetes as caused by a confused immune system, he suggests it is caused by stressed beta cells.
Slide depicting the current understanding on how type 1 diabetes develops. Source: Professor Bart Roep/ISPAD 2022 livestream
To explain this he cited the late Gian Franco Bottazzo, who promoted a radical idea: rather than the immune system causing indiscriminate destruction of beta cells (homicide), the beta cells are literally asking to be killed (as also happens with cancer cells) and the immune system is obliging.
Diabetes treatment: the case against immunosuppressants
When I was first diagnosed with type 1 diabetes and told it was a problem of the immune system, my first thought was to turn to immunosuppressive drugs, as we do with organ transplants to prevent the immune system from attacking the new organ. So why do we not use immunosuppressants to slow down the destruction of the pancreas? Because, in this case, the treatment is considered worse than the disease.
There are significant side effects from immunosuppressants, not the least of which is an increased risk of cancer. It turns out the body’s cells know they are cancerous, or infected by a virus, and literally put their hand up to be killed by the immune system. When we suppress the immune system, this goes unchecked; infections and cancers are left unhindered and compromise the host.
On the other side of the coin, if we boost the immune system, this can trigger a disease very similar to type 1 diabetes; the immune system becomes much more sensitive to stressed cells and attacks cells with less discrimination. Great for suppressing cancer, but not so great for beta cells.
Beta cells under stress: can reducing cell stress impact diabetes management?
So why are beta cells the outlier in being attacked? Why does the immune system focus its efforts here and not on the entire body? As it turns out, beta cells are some of the most hard working cells in the human body, producing literally a million copies of insulin every minute. It therefore makes sense that it would not take a lot to tip a beta cell over the stress threshold and cause it to be targeted by the immune system.
Slide depicting the role of the stressed beta cell in Professor Roep’s theory. Source: Professor Bart Roep/ISPAD 2022 livestream
Latent autoimmune diabetes of adults (LADA) forums frequently advise that to prolong the honeymoon phase (i.e. the time when beta cells are still producing some amount of insulin), you must reduce the stress on the beta cells. Recommendations often include dietary adjustments, such as eating low carbohydrate, supplements to reduce inflammation, and early-intervention insulin to reduce the demand on the beta cells.
Even in medical literature there is evidence (or, at least, consistency) that beta cell stress accelerates the destruction of beta cells. In “Latent Autoimmune Diabetes in Adults: A Review on Clinical Implications and Management”, a summary of findings from other studies concluded that:
insulin sensitizers plus insulin therapy can preserve beta cell function better than insulin alone,
progression to insulin dependence was slower when insulin therapy was used compared to the use of sulfonylureas (a class of drugs which force beta cells to produce more insulin),
and that weight loss can lower insulin resistance and preserve beta cell function, according to evidence presented in “Beta-Cell Preservation…Is Weight Loss the Answer?”.
All of the above findings are consistent with this stress model for type 1 diabetes. Similarly, this model does not exclude other things, such as viruses, as being involved: it simply recasts their role as stressors of the beta cells rather than antagonists of the immune system.
Can today’s cancer treatment inform the future of diabetes management?
Looking at cancer treatment breakthroughs and applying an inverse approach, we get a novel set of approaches for curing/managing type 1 diabetes.
Slide depicting current cancer treatments and how they could be used to treat type 1 diabetes in the future. Source: Professor Bart Roep/ISPAD 2022 livestream
CAR T cells
In cancer, the immune cells (T cells) are re-engineered to attack cancer cells. For type 1 diabetes, the re-engineering would be with the cells that regulate the T cells (Regulatory T cells) to suppress the immune response specifically for beta cell destruction.
Bionic: therapeutic Ab conjugated with toxin
In cancer, antibodies are engineered to carry a toxin so that when they attack the cancer cell, they deliver a toxic payload to wipe out that cell. This same technique would be used but to deliver a growth factor that would revitalise the beta cell.
Reduce hypo inflammatory tumour environment
Cancer cells actively reduce inflammation to limit the immune response. Treatments often seek to reverse this. In the case of type 1 diabetes, treatments would look to enhance the low inflammatory state.
DC vaccination (Dendreon)
In cancer, a company called Dendreon has a vaccine which selectively activates the immune system. For type 1 diabetes, we can selectively suppress the immune system through the use of vitamin D3.
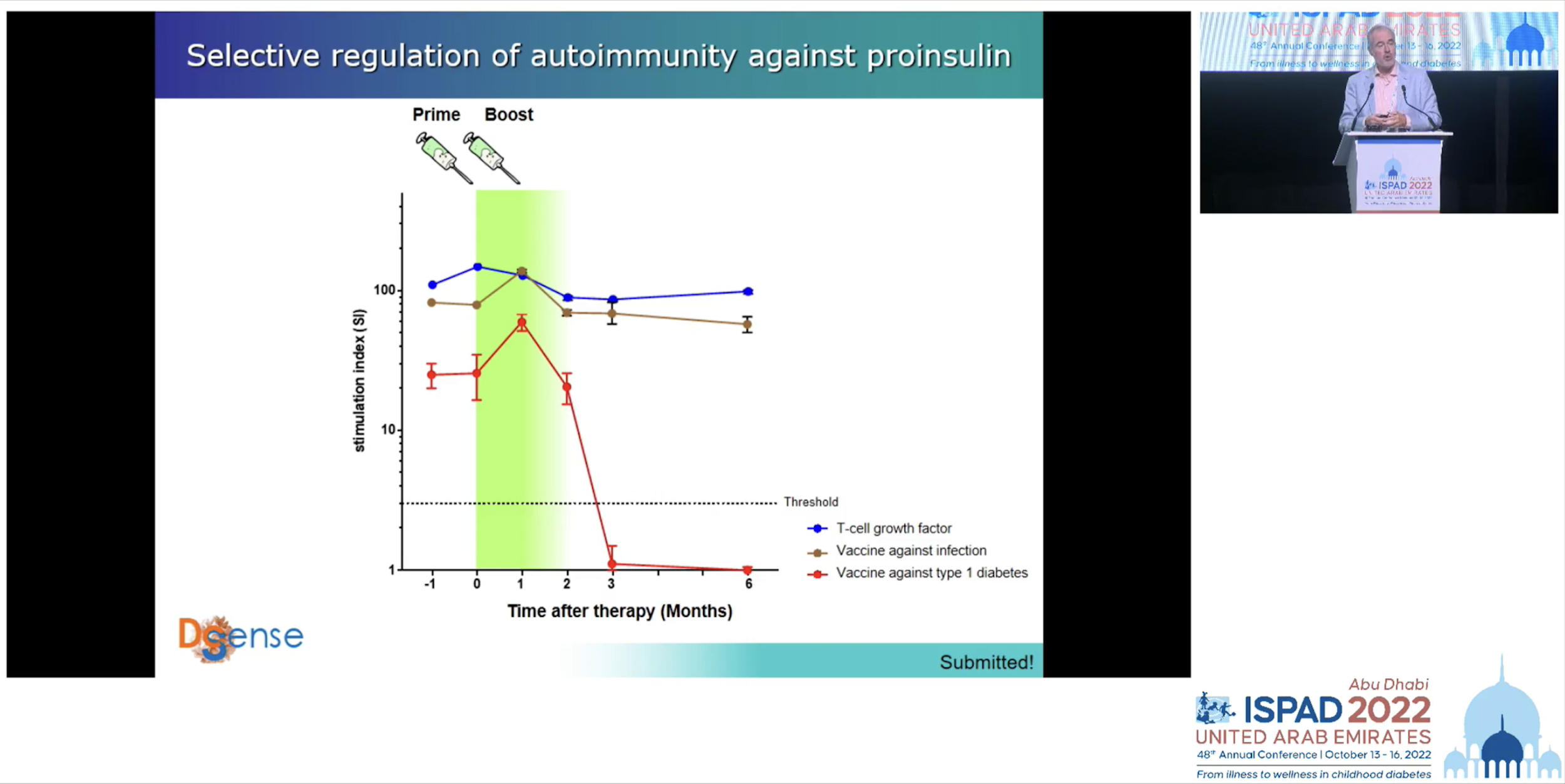
In the case of this last approach Professor Roep’s team has tested it with excellent results; the general immune system was preserved but the immune component responsible for attacking the pancreas was suppressed.
Slide depicting test results of a vaccination against type 1 diabetes. Source: Professor Bart Roep/ISPAD 2022 livestream
The future of diabetes treatment
Professor Roep emphasised that having a variety of treatments on offer means a treatment plan can be tailored to individuals, just as cancer drugs provide a set of tools in the toolkit of the oncologist with patients responding to different ones.
While I enjoyed the entirety of ISPAD 2022, this was, for me, the stand out presentation. Challenging the type 1 origin story, presenting a model which is consistent with a broad range of observations, and pioneering resulting therapies is, for me, what these conferences are all about. I look forward to seeing the progress of Professor Roep’s work in the future.
Found this article insightful? Please spread the word and share it with your network.
This article was originally published on Leon’s blog, The Practical Diabetic, on 27 March 2023.